renato
Member
Full Member
- Messages
- 68
- Reaction score
- 7
Pink has always been a hard color to achive uniformly with regularity in results. Desert Eagle quit offering pink anodized pistols for that reason.I am following this chart for voltage, I can get all colors solo including Pink.
The problem is making it bicolor the pink looks weird it is not even
View attachment 29479
I think it looks really dark... only really matters how it looks under the tissue. Dark purple on an implant is no bueno.. but maybe it matches well..

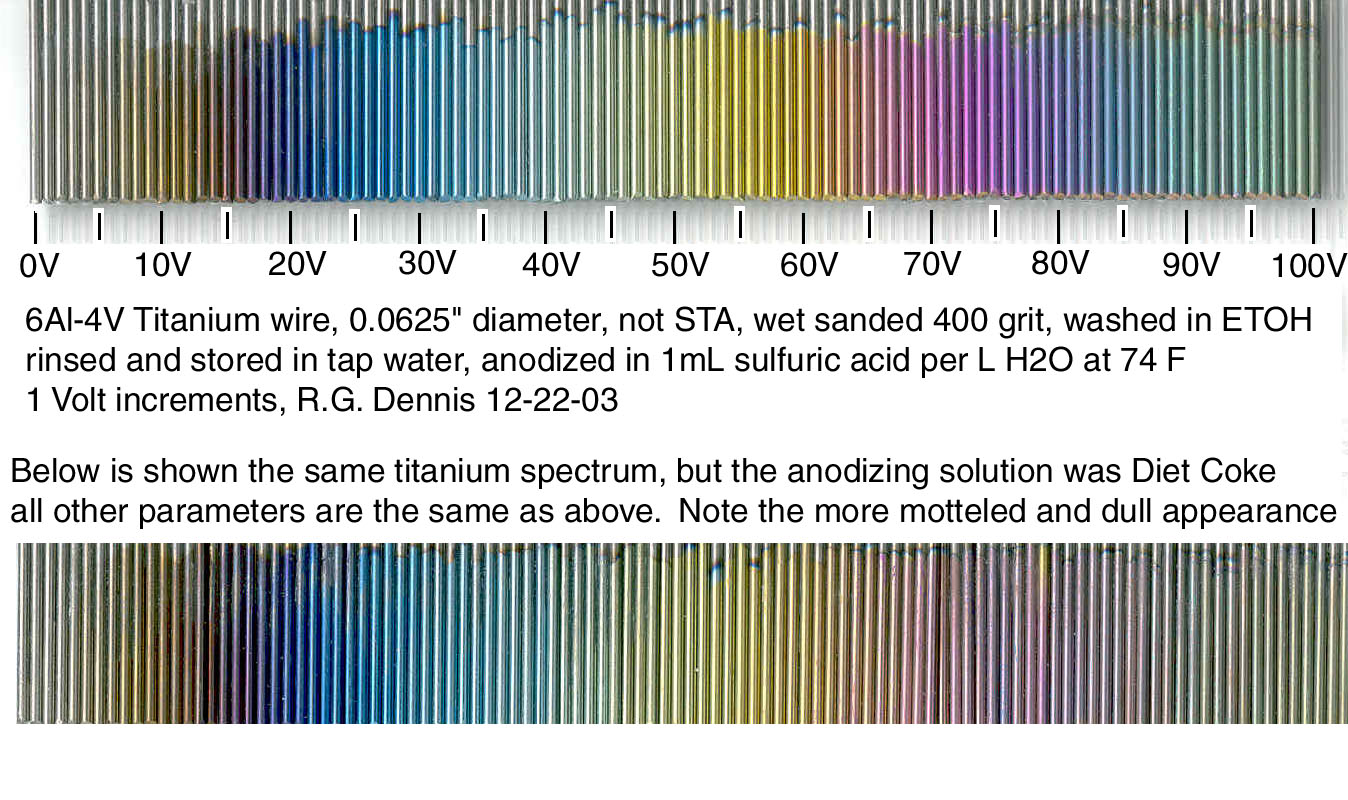
"tap water" being a huge variable in itself.it would be really helpful to know your solution too, most of the literature surrounding anodization suggests varying kinds of base solutions, with better results using pure bases such as sulfuric acid, or hydrofluoric acid at 1% in a controlled temperature tap water, with again varying results dependingon the composition of the cathode, and resistivity of the leads as well.
Mine has so much rust in it I cure in distilled.I wouldn’t use tap water at all, distilled is a must you don’t want any metals or chlorine like I have here.
Leave it in the fridge in an open container. It off gases and then is palatable.I quit drinking water at the lab because the odor of chlorine is sickening, I had to get bottled water to get any hydration. At home we have a great community water system, but still filter it through the fridge. #firstworldproblems

